Putting all your customers front and centre: Lean Quality Management.
Lean as a manufacturing philosophy has been an extremely successful manufacturing approach over the past 30 years. Today there are few successful organisations who have not at least attempted to integrate lean into their manufacturing systems to replicate the success of Japanese companies and gain a competitive advantage. Whilst it can be argued, by some, that the peak of lean has passed, even a quick Google search of “Lean Manufacturing “ yields over 9 million hits.
Key to the application of a lean approach is putting the customer central and identifying what is considered “Value” through the eyes of the customer, then eliminating waste to make the value-stream “Flow”.
So why has this methodology, so successful in manufacturing, been relatively overlooked in the Quality Management arena ? A similar search of “Lean Quality Management Systems “ yields only around 13,000 hits, many of these buried within the academic literature around Quality. So why the disparity with lean QMS having less than 0.2% the coverage of lean manufacturing ? I think the reasons are three-fold.
1: Lean is viewed as predominantly a manufacturing philosophy.
Rightly or wrongly lean is seen to be focussed on Operations and whilst there has been an explosion of application of lean methods in the service sector, lean is still generally viewed as an operational focussed set of tools. This does not have to be, and should not be the case.
2: The often blinkered definition of customers of the Quality System.
All Quality systems talk about customers, but generally this is the end customer of the product or service. Few look at who the customers of the QMS are and their specific needs. Whilst any QMS has multiple customers often the sole customer is seen as the regulatory or notifying bodies auditing the QMS against a standard or regulation. Perversely the QMS is often seen to exist solely to meet a regulatory need, rather than a framework for organisational advantage. Related to this, Quality functions within organisations are often not seen as drivers of organisational change and improvement but perform a more quality assurance or quality control role.
3: The QMS is primarily seen as a means to control product manufacture to a specification
Certainly within the Medical Device industry the relevant ISO standard - 13485: 2016 - has strong focus on ensuring regulatory compliance and safeguarding that the device meets customer expectations regarding performance and safety, with far lesser focus on continuous improvement compared with ISO9001:2015.
Taking these three areas in turn.
Firstly, any process where there is a customer who has expectations, lean can be applied. Even areas not directly related to the manufacturing process.
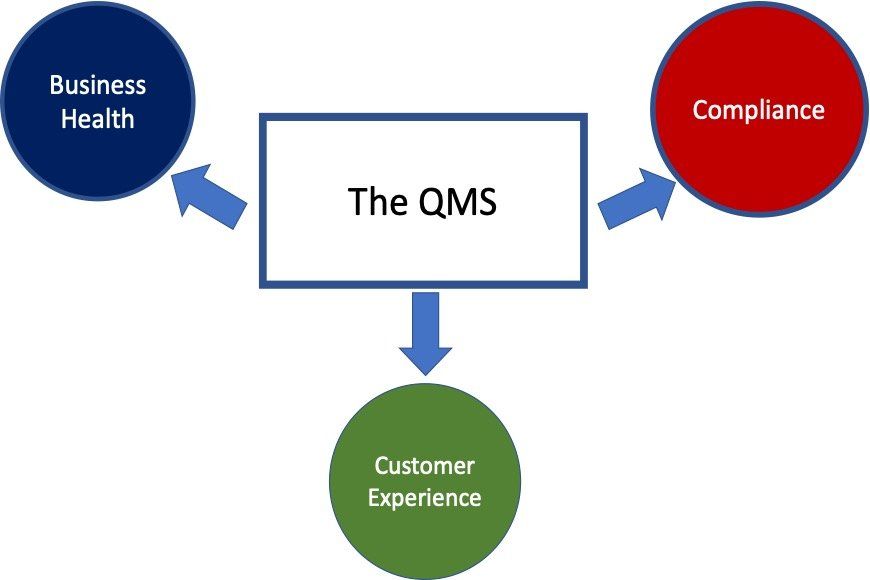
Secondly the QMS has many customers. These can be broadly placed into three groups: Compliance
-meeting a regulatory need; Business Health
-growth, profitability etc and lastly the Customer Experience
– everything from performance to delivery and cost.
These three different customers can often have very different views of “Value”, with perspectives all equally valid. Any QMS that focusses only on one aspect – eg: Compliance – is unbalanced and not meeting its potential.
Thirdly, whilst ISO13485 is focussed on regulatory compliance any organisation that doesn’t continually question and improve is left behind. Quality organisations play a key role in making this happen. End customers don’t buy products primarily for compliance reasons. Related to this, expectations are also changing. The Medical Device Regulations (MDR) which all medical device manufacturers must legally follow by 2021 to market products in the EU, stipulates:-
“Manufacturers of devices…shall establish, document, implement, maintain, keep up to date and continually improve a quality management system. “
Simply maintaining a QMS will no longer be good enough.
So now is time for Quality Systems leaders to start incorporating methodology to continually improve the QMS for reasons of both organisational advantage and to meet regulatory mandates that are coming.
Datod
has just released a frame-work and associated methodology that details a process to derive Key Performance Indicators directly linked back to multiple Customer’s view of “Value” of the QMS.
This methodology can be used to develop detailed and joined up Quality Objectives, to not only meet the regulatory obligations that are coming, ensure that the QMS operates effectively and efficiently for all its customers, but also ensure it generates a real competitive advantage for your business.